For your patients managing recurring cystine stone events, consider
THIOLA EC
If your patient is still experiencing recurring stones under his or her current treatment plan, THIOLA EC may help.
INDICATIONS & USAGE: THIOLA EC is indicated, in combination with high fluid intake, alkali, and diet modification, for the prevention of cystine stone formation in adults and pediatric patients ≥20 kg with severe homozygous cystinuria, who are not responsive to these measures alone.
*Copay Terms and Conditions:
- Program is valid for patients with commercial or private insurance
- Must be a US resident
- Not valid for patients insured by a federal or state government-funded health plan, including Medicare, Medicare Advantage, Medicaid, and TRICARE
- Void where the program is prohibited by law
- Not valid for uninsured patients
- Program does not replace prescription drug coverage or insurance and is not intended to substitute for coverage
- Travere reserves the right to terminate or modify this program at any time without notice
71% of patients achieved stone prevention with tiopronin in a multi-center clinical trial1
| Efficacy results in patients with cystinuria1 |
Naïve to d‑penicillamine |
|---|---|
Stopped new stone formation |
71% |
Reduced rate of new stone formation |
94% |
In patients previously treated with d-penicillamine (n=43)1:
- 62% stopped forming new stones
- 81% reduced the rate of new stone formation
Study design: Sixty-six cystine stone-formers (mean age 32.0±13.3 years), with 1 or more stone episodes (spontaneous passage, surgical removal, or appearance on X-ray) in the prior 2 years, were enrolled at 12 study sites. Forty-nine patients had been previously treated with d-penicillamine (at a mean dosage of 1125±640 mg/day for a mean of 2.81±3.94 years) and 17 patients had no prior d-penicillamine therapy. All patients maintained ongoing dietary and fluid regimens, and 45/66 patients continued ongoing alkali therapy. Patients received tiopronin for between 4 months and 4 years at a mean dosage of 1193±450 mg/day, in 3 to 4 divided doses ≥1 hour before or 2 hours after meals.1
THIOLA EC may help prevent stones by binding to cystine2
Prevention of stones requires removal of excess cystine
Tiopronin increases solubility of cystine
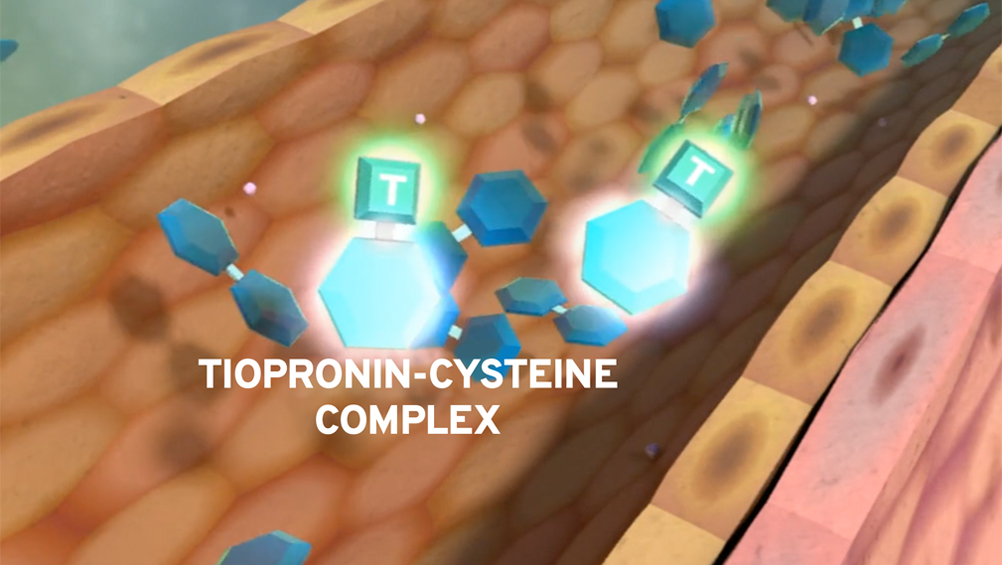
Tiopronin undergoes thiol-disulfide exchange with cystine, forming a more soluble complex.
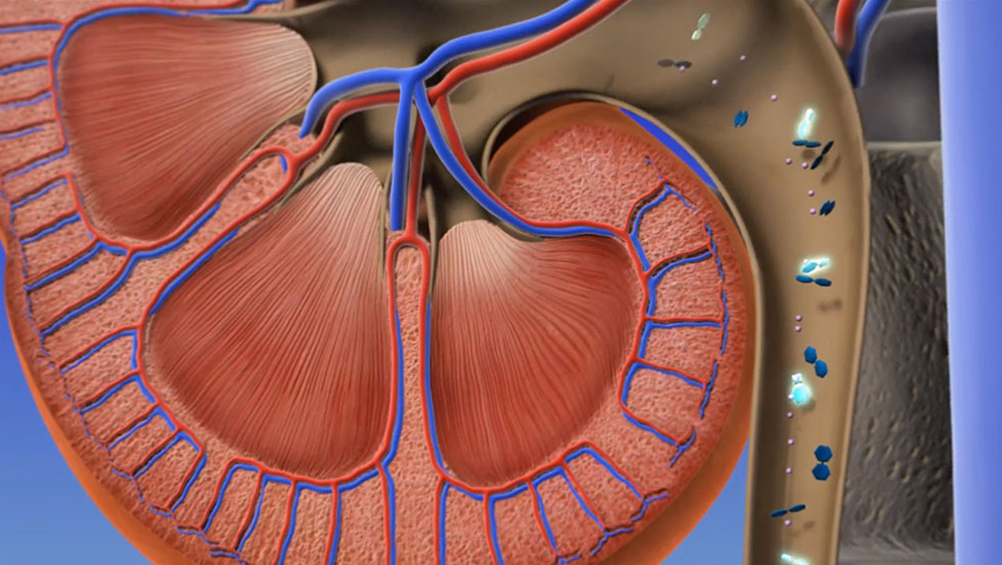
Tiopronin-cysteine complex is excreted in the urine, reducing the possibility of stone formation.
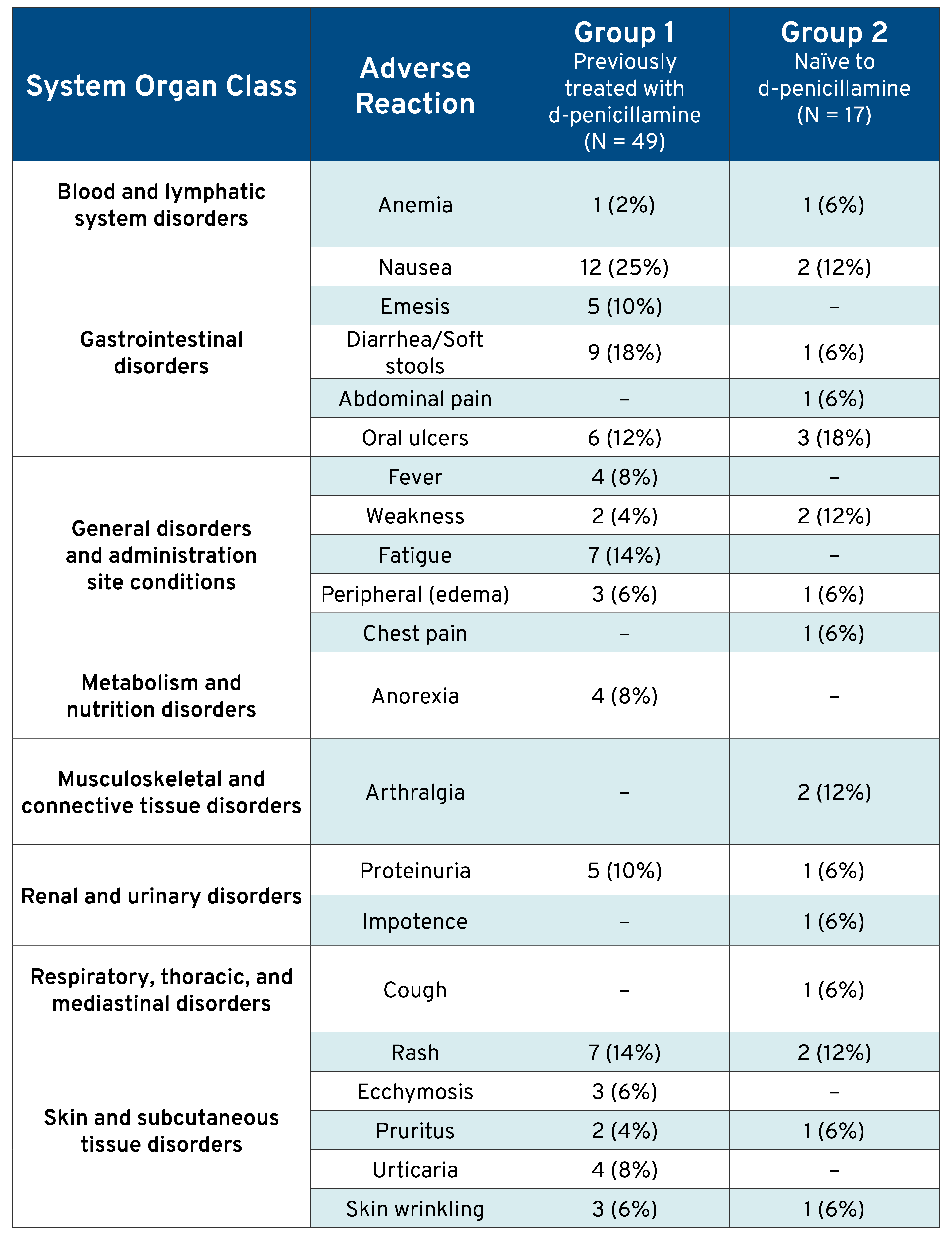
Adverse reactions (≥5%) occurring in a multi-center trial of 66 cystine stone-formers treated with THIOLA1,2
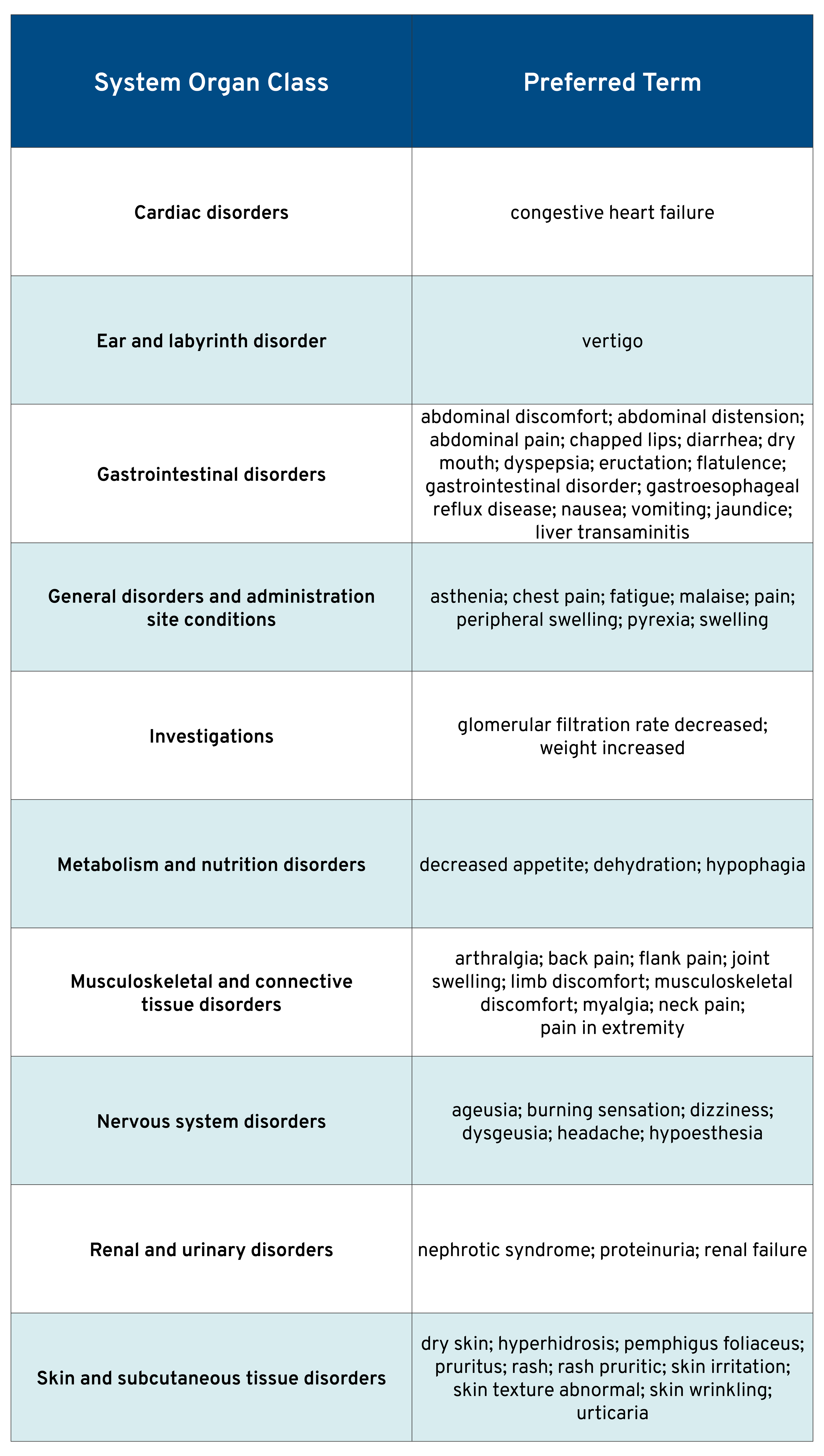
Postmarketing adverse reactions reported2
Routinely monitor cystine levels with 24-hour urine tests to find the right dosage for your patients.1,5
References: 1. Pak et al. J Urol. 1986;136(5):1003-1008. 2. THIOLA EC [package insert]. San Antonio, TX: Mission Pharmacal Company. 3. Pareek et al. J Urol. 2005;174(6):2250-2252. 4. Pearle et al. J Urol. 2014;192(2):316-324. 5. Knoll et al. Pediatr Nephrol. 2005;20(1):19-24.
THIOLA EC® (tiopronin, delayed-release tablets)
INDICATIONS AND USAGE: THIOLA EC® (tiopronin, delayed-release tablets) is indicated, in combination with high fluid intake, alkali, and diet modification, for the prevention of cystine stone formation in adults and pediatric patients ≥20 kg with severe homozygous cystinuria, who are not responsive to these measures alone.
Important Safety Information
CONTRAINDICATIONS: THIOLA EC is contraindicated in patients with hypersensitivity to tiopronin or any other components of THIOLA EC.
WARNINGS AND PRECAUTIONS:
- Proteinuria: Proteinuria, including nephrotic syndrome, and membranous nephropathy, has been reported with tiopronin use. Pediatric patients receiving >50 mg/kg of tiopronin per day may be at increased risk for proteinuria. Monitor patients for the development of proteinuria and discontinue therapy in patients who develop proteinuria.
- Hypersensitivity Reactions: Hypersensitivity reactions (drug fever, rash, fever, arthralgia and lymphadenopathy) have been reported.
ADVERSE REACTIONS: The most common adverse reactions (≥10%) are nausea, diarrhea or soft stools, oral ulcers, rash, fatigue, fever, arthralgia, proteinuria, and emesis.
DRUG INTERACTIONS: Avoid alcohol consumption 2 hours before and 3 hours after taking THIOLA EC as THIOLA EC is released faster in the presence of alcohol.
SPECIFIC POPULATIONS:
- Lactation: Breastfeeding is not recommended during treatment with THIOLA EC.
- Geriatric Use: Because elderly patients are more likely to have decreased renal function, care should be taken in dose selection, and it may be useful to monitor renal function.
Please click here for full Prescribing Information and additional Important Safety Information.
THIOLA® (tiopronin tablets)
INDICATIONS AND USAGE: THIOLA® (tiopronin tablets) is indicated, in combination with high fluid intake, alkali, and diet modification, for the prevention of cystine stone formation in adults and pediatric patients ≥20 kg with severe homozygous cystinuria, who are not responsive to these measures alone.
Important Safety Information
CONTRAINDICATIONS: THIOLA is contraindicated in patients with hypersensitivity to tiopronin or any other components of THIOLA.
WARNINGS AND PRECAUTIONS:
- Proteinuria: Proteinuria, including nephrotic syndrome, and membranous nephropathy, has been reported with tiopronin use. Pediatric patients receiving >50 mg/kg of tiopronin per day may be at increased risk for proteinuria. Monitor patients for the development of proteinuria and discontinue therapy in patients who develop proteinuria.
- Hypersensitivity Reactions: Hypersensitivity reactions (drug fever, rash, fever, arthralgia and lymphadenopathy) have been reported.
ADVERSE REACTIONS: The most common adverse reactions (≥10%) are nausea, diarrhea or soft stools, oral ulcers, rash, fatigue, fever, arthralgia, proteinuria, and emesis.
SPECIFIC POPULATIONS:
- Lactation: Breastfeeding is not recommended during treatment with THIOLA.
- Geriatric Use: Because elderly patients are more likely to have decreased renal function, care should be taken in dose selection, and it may be useful to monitor renal function.
Please click here for full Prescribing Information and additional Important Safety Information.
